双键
作者:上海滩小药师
为什么要买这个呢?当然是要先货比三家
因为家里都是小米的产品,所以如果想换的话,只能换小米的家居产品,没有办法没有选择
1.货比三家
这次选了一个无线开关
因为阳台上没有接线的地方,所以只能选择一个用电池的无线开关
2.开箱晒物
下面开始开箱了


包装盒是最小的

目前看过最小的

用的是Cr2032电池

一般能用个一两年吗?感觉一年肯定是没问题

主要还是要看家里的无线环境

之前买过一个放在阳台上被压住了,最后一天电就没了
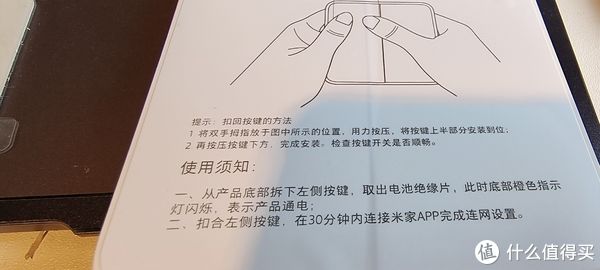
撕开表面


里面的电池上面有个胶套,把它去掉就可以用

话说这里要提醒一下,这个盖子真难开

也没有说明,其实就是硬抠

不过硬抠了又可以,可能导致这个盖子损坏

接着要连接手机APP

米家APP

这是蓝牙连接

据说现在蓝牙连接比较省电

开始设置功能
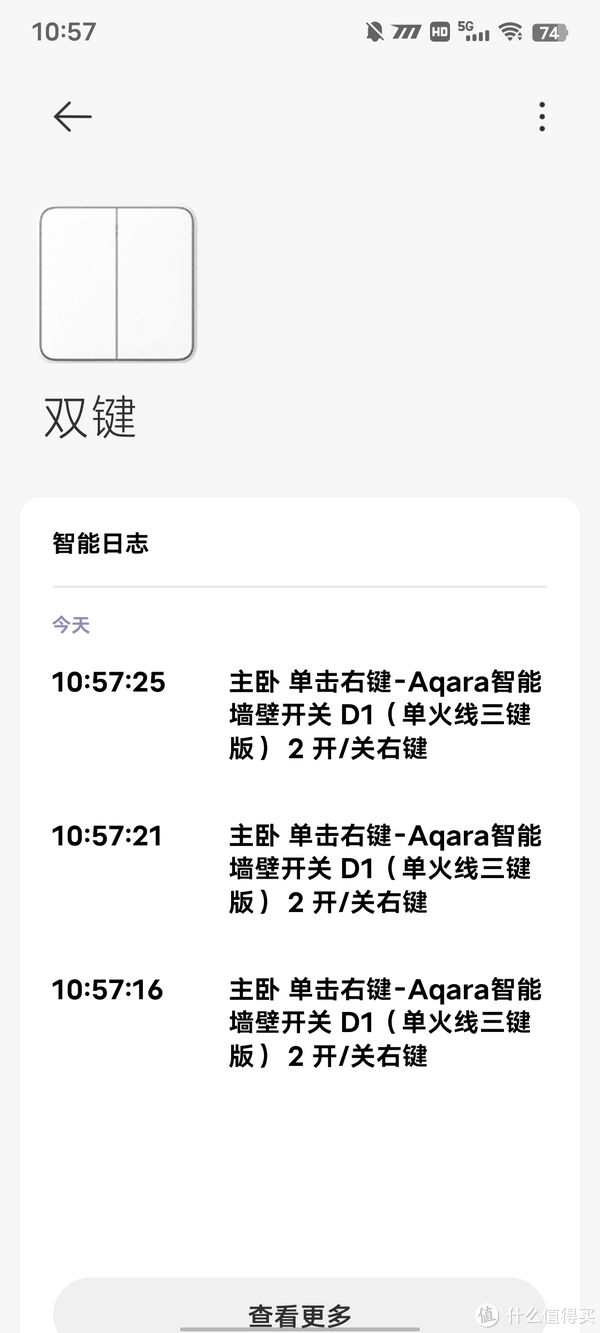
可以把左边设置为电脑的插线板,右边设置为阳台的灯

随便贴在柜子上,还是很方便的
3.产品说明
我们来看一下他的产品说明吧,为了以后方便查找
这里分享的是产品说明书



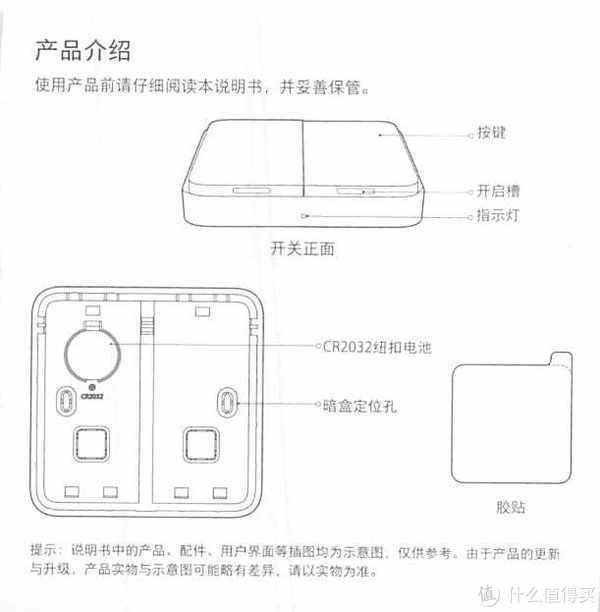
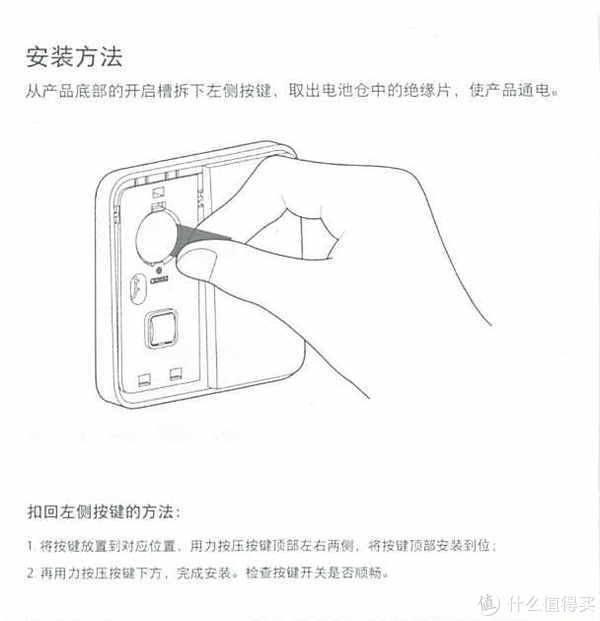

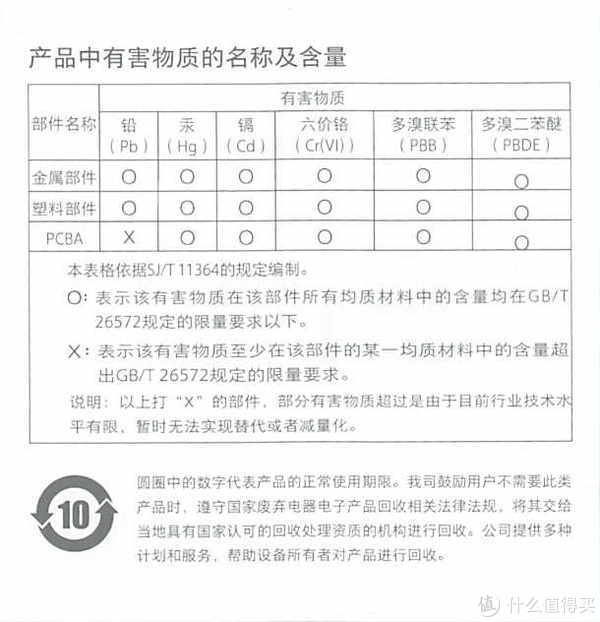
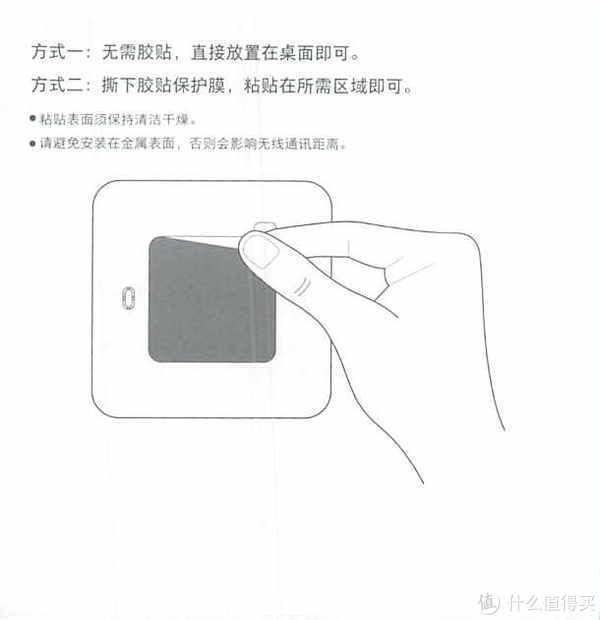
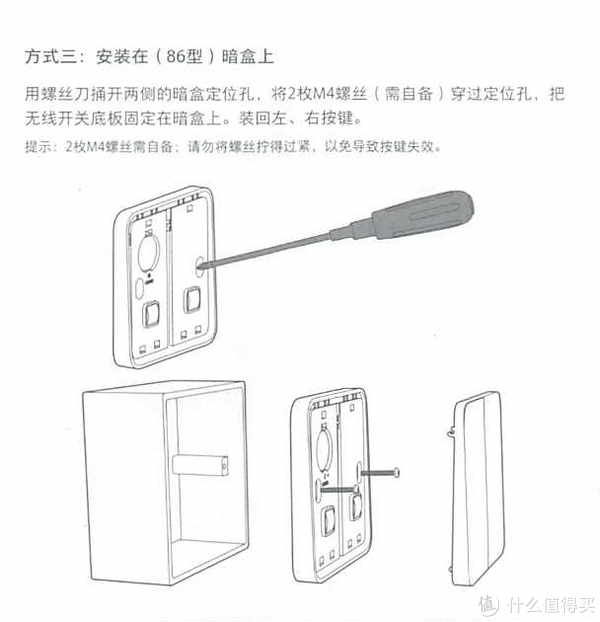




为什么要放产品说明书呢?因为时间长了自己也忘了怎么使用了。
偶尔回来翻翻还挺好
4.总结一下
(1)小米的蓝牙无线开关具有很高的功能性。它可以通过手机应用远程控制,非常方便。另外,它还具有定时开关、语音控制和场景联动等功能,能够满足用户多种使用需求
(2)这种蓝牙无线开关易于使用。用户只需下载小米智能家居应用,连接设备,即可使用。另外,它的配对和安装也很简单,不需要专业技能。缺点就是要配合蓝牙网关,使用附近一定要有网关,而且不能太远。
(3)开关设计简约,外观美观。它采用白色塑料外壳,与大多数墙壁的颜色相似,不会破坏家庭装饰的美感。另外,它的尺寸小巧,可以与墙壁紧密结合,不占用空间。关键是价格也不算太贵。

最后,大家打赏,收藏,评论请随意,但请点个赞,点赞功能就是文章左下角的大拇指标签
评论区有你更精彩,你们动动手指,对我可是意义重大(*ワ*)
","gnid":"9404cea694a97079a","img_data":[{"flag":2,"img":[{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01302e3e81b81861d9.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01ca2240ed905c6df1.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t019264327edf072a12.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t018ff49d81fee9c945.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01940742c086fb46f3.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01a9ea17fb1aa1cdf4.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01c5f02d169760b640.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01a58d159cb72db83f.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t0192a5266a53579233.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01aa3c1081e96e8514.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01727d248c7883bf89.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t0195feb3eae69596e9.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01d6f769552e7e7a00.jpg","width":"600"},{"desc":"","height":"1333","title":"","url":"https://p0.ssl.img.360kuai.com/t01d4f413866e2041d4.jpg","width":"600"},{"desc":"","height":"1333","title":"","url":"https://p0.ssl.img.360kuai.com/t018f2562a2d42ab8b5.jpg","width":"600"},{"desc":"","height":"1333","title":"","url":"https://p0.ssl.img.360kuai.com/t019bbe3c23e7aa232e.jpg","width":"600"},{"desc":"","height":"1333","title":"","url":"https://p0.ssl.img.360kuai.com/t015c5dad161bb13db6.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01a5265de0677f7c1f.jpg","width":"600"},{"desc":"","height":"1333","title":"","url":"https://p0.ssl.img.360kuai.com/t017f7783d9917b4a79.jpg","width":"600"},{"desc":"","height":"270","title":"","url":"https://p0.ssl.img.360kuai.com/t01fc43a209334bc249.jpg","width":"600"},{"desc":"","height":"621","title":"","url":"https://p0.ssl.img.360kuai.com/t0129ac22a9c833b2ab.jpg","width":"600"},{"desc":"","height":"616","title":"","url":"https://p0.ssl.img.360kuai.com/t01686aaa0ddcb38b4e.jpg","width":"600"},{"desc":"","height":"609","title":"","url":"https://p0.ssl.img.360kuai.com/t01f23ec42395c3f929.jpg","width":"600"},{"desc":"","height":"612","title":"","url":"https://p0.ssl.img.360kuai.com/t01c2588d04e7bf73ae.jpg","width":"600"},{"desc":"","height":"621","title":"","url":"https://p0.ssl.img.360kuai.com/t013d6b0ad8b3ee06dd.jpg","width":"600"},{"desc":"","height":"616","title":"","url":"https://p0.ssl.img.360kuai.com/t017fa1966489aa26ab.jpg","width":"600"},{"desc":"","height":"622","title":"","url":"https://p0.ssl.img.360kuai.com/t01ca23bd3bb7e26adf.jpg","width":"600"},{"desc":"","height":"620","title":"","url":"https://p0.ssl.img.360kuai.com/t01d62375388a8fb1ea.jpg","width":"600"},{"desc":"","height":"622","title":"","url":"https://p0.ssl.img.360kuai.com/t0197a9e97e6886a59e.jpg","width":"600"},{"desc":"","height":"615","title":"","url":"https://p0.ssl.img.360kuai.com/t0163ece0a638cbd499.jpg","width":"600"},{"desc":"","height":"617","title":"","url":"https://p0.ssl.img.360kuai.com/t01132be62367c148df.jpg","width":"600"},{"desc":"","height":"622","title":"","url":"https://p0.ssl.img.360kuai.com/t01304a38509297c6e1.jpg","width":"600"},{"desc":"","height":"614","title":"","url":"https://p0.ssl.img.360kuai.com/t01ac7f57b33b4d9f8d.jpg","width":"600"},{"desc":"","height":"302","s_url":"https://p0.ssl.img.360kuai.com/t01988e671de5d04cfc_1.gif","title":"","url":"https://p0.ssl.img.360kuai.com/t01988e671de5d04cfc.gif","width":"360"}]}],"original":0,"pat":"xmc,art_src_1,fts0,sts0","powerby":"hbase","pub_time":1681739916000,"pure":"","rawurl":"http://zm.news.so.com/b2481bc42f98b63bf6cc733f061d3ffc","redirect":0,"rptid":"b3263ae715134b4d","rss_ext":[],"s":"t","src":"什么值得买","tag":[{"clk":"kdigital_1:app","k":"app","u":""}],"title":"居家维修厮 篇五十:可以随意粘贴的无线双键开关/家庭装修,补漏洞产品/小米无线开关双键版 米家智能联动 小爱控产品说明书
孔邹倩1173如何区别三键双键 -
甄习怀13225075283 ______[答案] 数一数两原子之间的公用电子对数目,有两对就是双键如CO2,有三对就是三键如CO. 主要是公用电子对数目的不同. 氮气就是氮氮三键,乙烯中就有碳碳双键
孔邹倩1173化学 单键双键叁键是什么意思?怎么判断? -
甄习怀13225075283 ______ 每个化学式中的原子最外层电子数为8时(氢为2),该分子才能稳定.氢原子最外层只有一个电子,2个氢原子各提供一个电子,配对成共价单键,形成氢分子.氧最外层为6电子,达到8电子的稳定状态,还需要两个,故每个氧原子提供2个电子形成共价双键,同理,氮最外层5电子,达到8还需要3个电子,所以形成3个配对的共价键,三键.
孔邹倩1173单键.双键.西格玛键.π键有何不同 -
甄习怀13225075283 ______[答案] 单键、双键是主要在有机化学中的说法,指碳原子之间的两种成见形式; 派、西格玛键,是化学价键理论中的说法: 原子的外层电子是呈一定电子云的形态分布的,如果只讨论p层电子在简单情况(两原子)下的成键,那么在成键时,可能有以下两...
孔邹倩1173有机物中的双键表现氧化性还是还原性? -
甄习怀13225075283 ______[答案] 含双键的分子中双键是由两个 产生的,由于电子对在两个原子之间没有明显的偏移,在原子上则表现为对其束缚作用不强,对强 物质来说,他表现出得电子的能力,对强氧化性物质来说则表现出失去电子的趋势,于是它的 相对活泼.换个词就是不稳...
孔邹倩1173孤立双键是什么啊,如碳碳双键键长0.134nm,(孤立双键键长0.133), -
甄习怀13225075283 ______[答案] 孤立双键是与共轭双键相对应的概念,双键其实包括一个σ键和一个π键,一对共轭双键的π键是连在一起的,孤立双键的则单独存在. ps:不存在一个共轭双键的说法,只能说一对乃至一系列共轭双键.
孔邹倩1173双键是哪个?
甄习怀13225075283 ______ 是双键盘吧!是左手控制R、D、V、G右手控制移动光标、(也就是普通玩法时所按的);又或者是左手控制移动光标,右手控制2、4、6、8
孔邹倩1173有机化学的单键双键叁键是什么 -
甄习怀13225075283 ______ 单键,在化合物分子中两个原子间以共用一对电子而构成的共价键.通常用一条短线“-”表示.如甲烷CH4、乙烷(CH3CH3)分子中的键. 在化合物分子中两个原子间以二对共用电子构成的重键叫双键. 在化合物分子中两个原子间以三对共用电子构成的重键叫叁键. 双、叁键化合物具有不饱和性,能发生加成、聚合等反应.
孔邹倩1173双键到底有几个化学键!还有硅的化学键数目来个老师 -
甄习怀13225075283 ______[答案] 看他最外层电子个数,有四个电子的话,且有三个其他原子连接,则一定有一个双键,因为多了一个电子要成键,当有n个键时,就有n减一个派键和一个C个码键
孔邹倩1173双键是吸电子基团吗
甄习怀13225075283 ______ 双键是吸电子基团.有机化学中取代基效应分为四类:诱导效应、共轭效应、超共轭效应和场效应.前两者最常见,一个取代基究竟是吸电子效应还是给电子效应,看这两个效应的综合(当然有些基团不存在共轭效应).双键是共价键的一种,共价键,就意味着共用电子对的存在.简单的说,就是这一对电子,由键的两方各出一个,彼此共用.因此,一个共价键就可以填补一个最外层电子的空额.
孔邹倩1173化学中的单键和双键是什么意思??
甄习怀13225075283 ______ 单键就是两个原子共用一对电子对 双键就是两个原子共用两对电子对